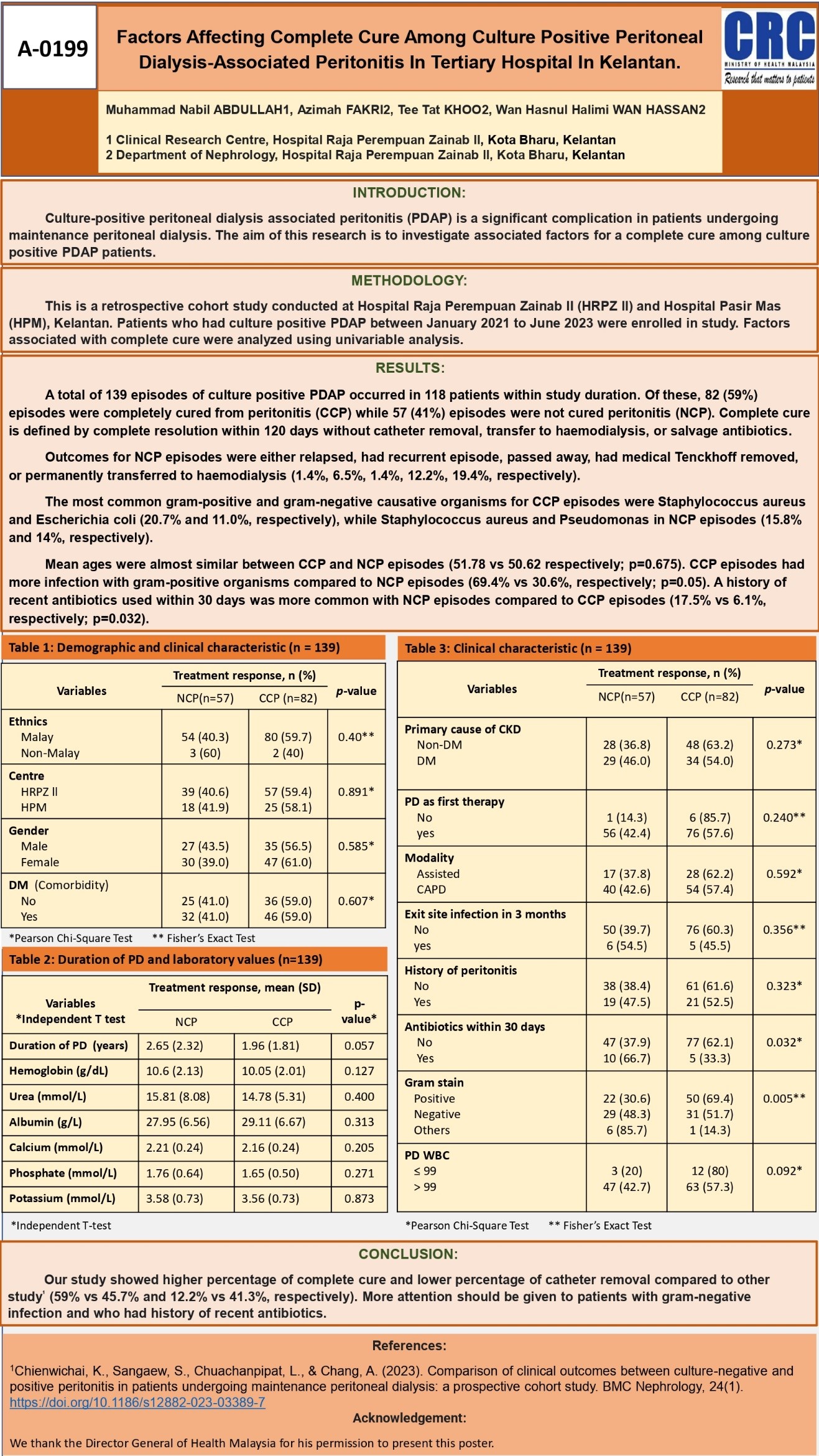
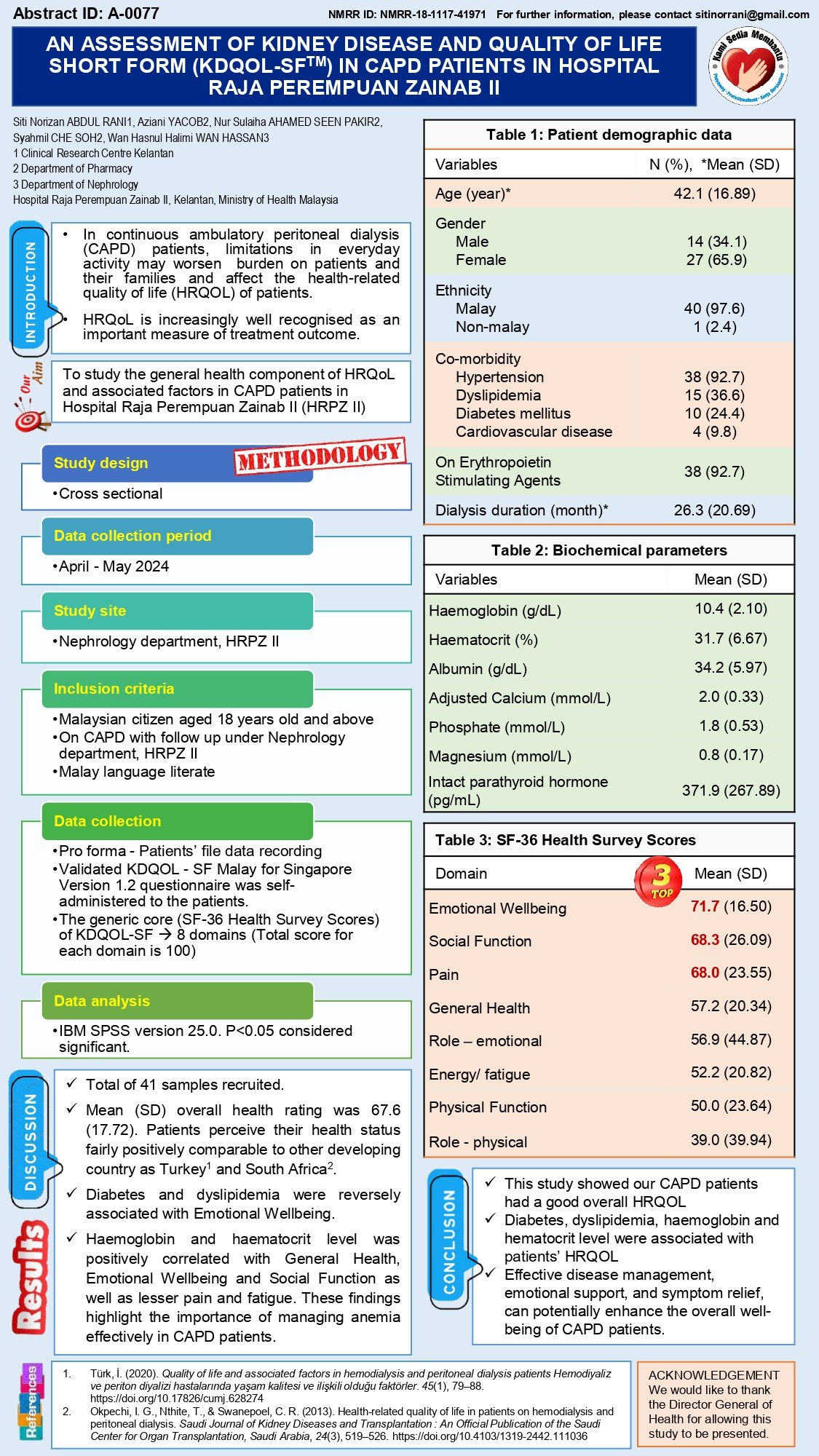
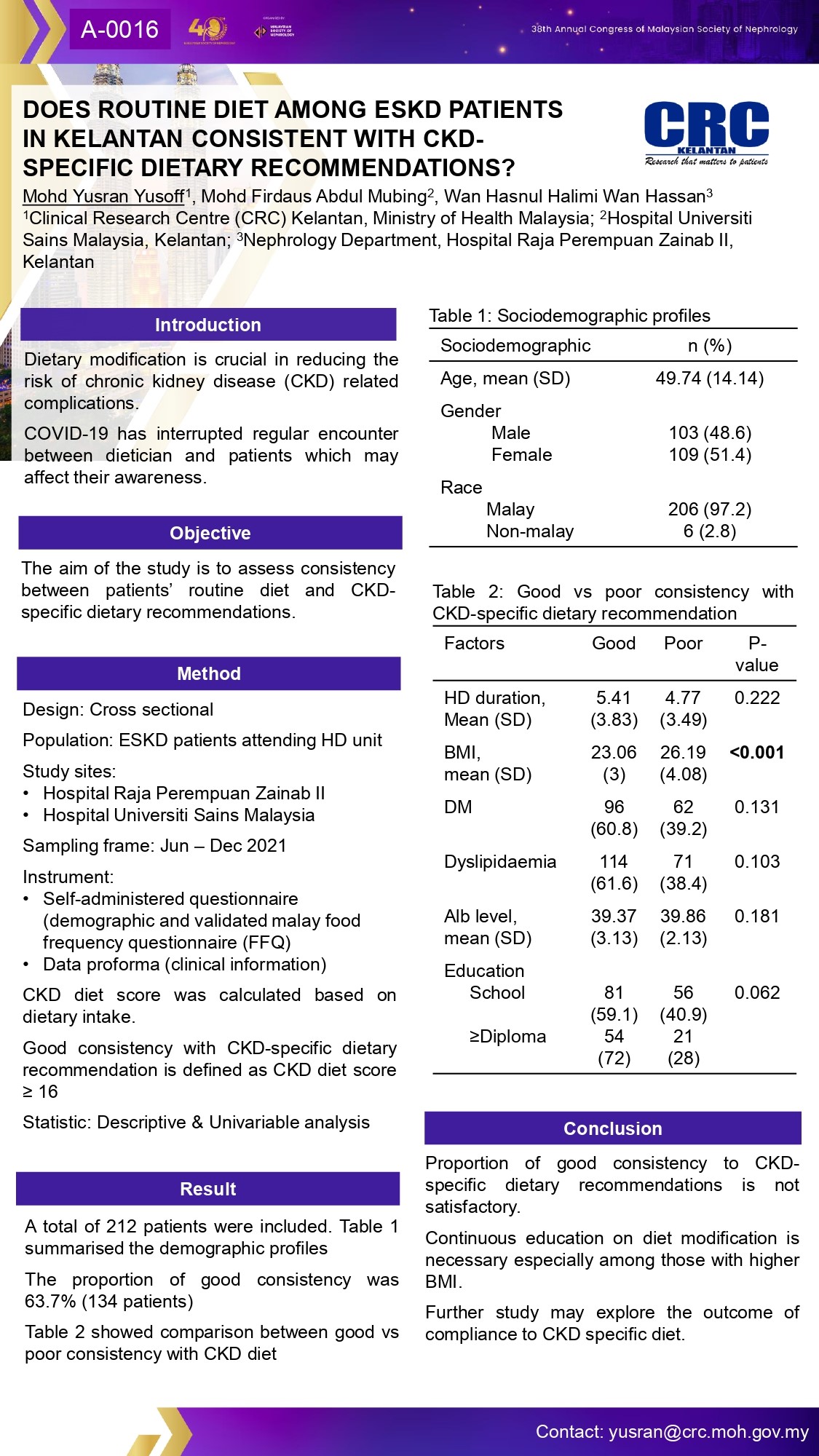
Clinical Research Centre
Hospital Raja Perempuan Zainab II
Kota Bharu, Kelantan
WhoWe Are
Clinical Research Centre Hospital Raja Perempuan Zainab II (CRC HRPZ II) which has been operating since 2007, functions as the clinical research arm of the National Institutes of Health, Ministry of Health Malaysia. It is one of the 37 network CRCs across the country with the primary role of promoting, supporting and conducting clinical research. With manpower of 9 staff, CRC functions as a provider of integrated clinical trial services to researchers in public institutions and industries. Supported by information technology capabilities, CRC is recognized for its track record in completing clinical studies on time, within the budget and to the highest ethical standards and, where required, in compliance with Good Clinical Practice (GCP) and applicable regulatory guidelines. CRC also provides research-related courses for investigators and aspiring researchers. Research Consultation Clinics (RCC) are regularly offered by this centre as a service for clinicians working in the MOH to assist them in clinical research conduct. CRC Kelantan also has 8 staff from Clinical Research Malaysia (CRM) including 1 Associate Regional Manager who work closely with CRC since year 2013 to achieve its mission and vision. On a wider scale, the increasing number of clinical trials and trial sites will create more opportunity for health professionals to become researchers.
OurTeam
InvestigatorsHighlight

Head of CRC, Consultant Physician & Nephrologist, Head of Nephrology Department, Hospital Raja Perempuan Zainab II
Email: zienul3@gmail.com
MD, MMed (USM), Fellowship in Nephrology, National Specialist Register Certification (Malaysia)
Areas of interest: Nephrology, Internal Medicine, Transplant Medicine, Hypertension and Cardiovascular Health, Pulmonary Medicine, and Clinical Research
Dr. Wan Hasnul Halimi Wan Hassan is a distinguished Consultant Physician and Nephrologist with extensive experience in nephrology and internal medicine. He serves as the Head of the Department of Nephrology at Hospital Raja Perempuan Zainab II (HRPZ II) in Kota Bharu, Kelantan, and is also the Head of Nephrology Services for the state. As a committed educator, he has held lecturer roles at multiple universities and is involved in various national and international medical societies. His research includes notable work on kidney diseases and hypertension, earning recognition in the nephrology field.
Research Highlights
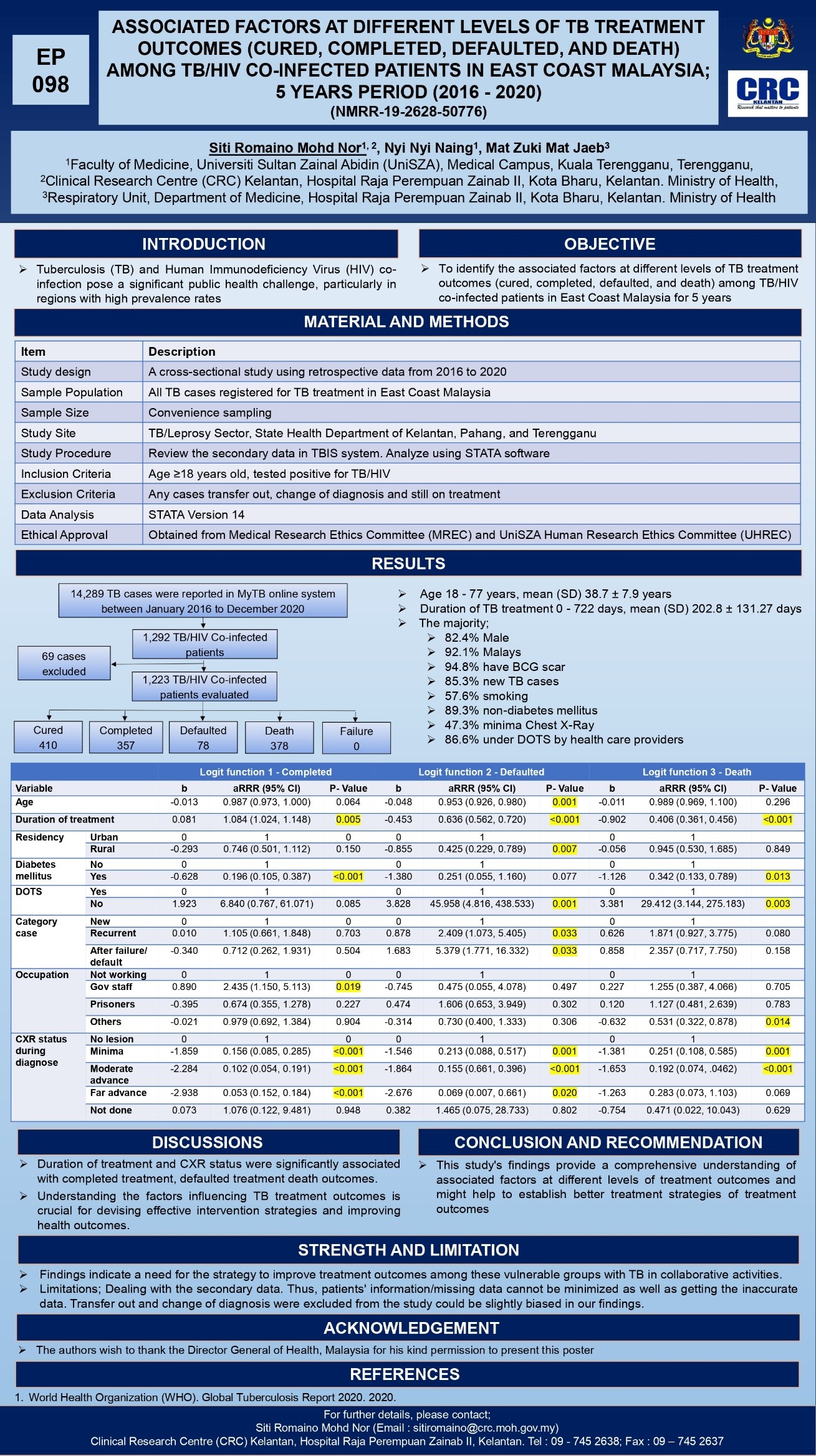
- Chronic Kidney Disease (CKD) Management: Analysis of ACE inhibitors in CKD patients, winning the 1st Prize at the National Hypertension Society Meeting (2010).
- Iron Management in Hemodialysis: A five-year audit on iron prescription patterns in Kelantan.
- Peritoneal Dialysis Infections: Investigating rapidly growing mycobacterium infections in dialysis patients.
- Blood Pressure Control Studies: Comparing diabetic and non-diabetic patients.
Recent Publications
Adult Polycystic Kidney Disease in patients on dialysis in Malaysia
Predialysis Clinic evaluation: Single Centre Experience
The Use Of ACE Inhibitors, ACE-I, ARB or both in Advance CKD Patients : ASingle Nephrology Centre Audit
The pattern of iron prescription and serum ferritin level among chronic haemodialysis patients in Kelantan: Afive year audit study.
A Cluster of Exit-site infection and Peritonitis involving Rapidly Growing Non Tuberculous Mycobacterium in Continuous Ambulatory Peritoneal Dialysis Patients
Non traumatic rhabdomyolysis following cholera infection. A case report
Comparison of blood pressure control in diabetic and non-diabetic patients
Project Highlights
| NMRR-23-00181-LIX | Randomised, double-blind, placebo-controlled, Phase IIb/Phase III study to evaluate the efficacy and safety of spesolimab in patients with moderate to severe hidradenitis suppurativa. Lunsayil 1. |
| NMRR-23-01937-CT2 | An Open-label, Multicenter, Single-arm, Post-marketing Trial (in Select Countries) to Evaluate Efficacy and Safety and the Impact of Immunogenicity on Efficacy, Safety, and Pharmacokinetics of Spesolimab i.v. in Treatment of Patients With Generalized Pustular Psoriasis Presenting With a Recurrent Flare Following Their Initial GPP Flare Treatment With Spesolimab i.v. |
| NMRR-24-00682-HGY | Lunsayil LTE: An Extension Trial Assessing Long-term Spesolimab Treatment in Patients With Hidradenitis Suppurativa (HS) |
| NMRR-20-824-54455 | A Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy and Safety of Odevixibat (A4250) in Children with Biliary Atresia Who Have Undergone a Kasai Hepatoportoenterostomy |
| NMRR-24-00686-H2S | A Randomized, Double-Blind Study to compare Efficacy, Safety and Pharmacokinetics between ABP 234 and Pembrolizumab (Keytruda) in Subjects with Advanced or Metastatic Non-squamous Non-Small Cell Lung Cancer |
| NMRR-21-1918-61166 | A Phase 3, Long-Term Extension Study to Evaluate the Safety and Efficacy of Imsidolimab (ANB019) in the Treatment of Adult Subjects with Generalized Pustular Psoriasis |
| A Phase 3 Study of Adjuvant Amivantamab and Lazertinib Combination versus Single-Agent Osimertinib in Patients with Evidence of Minimal Residual Disease after Resection of EGFR-Mutated Non-Small Cell Lung Cancer | |
| NMRR-23-02547-EHN | A Phase 2, Open-label Study to Assess the Safety and Efficacy of Bemnifosbuvir (BEM) and Ruzasvir (RZR) in Subjects With Chronic Hepatitis C Virus (HCV) Infection |
| NMRR-23-01871-OBV | A Randomized, Double-Blind, Parallel Group, Multicenter 12 Week Study to Assess the Efficacy and Safety of Budesonide and Formoterol Fumarate Metered Dose Inhaler Relative to Budesonide Metered Dose Inhaler in Participants With Inadequately Controlled Asthma (LITHOS) |
| NMRR-23-01017-JKC | Effectiveness and impact on quality of life of a combination of trimetazidine with one hemodynamic agent (ß-blocker or Ca-channel blocker), in patients recently diagnosed with stable angina and still symptomatic despite first line hemodynamic therapy: a prospective, international, non-interventional study |
| NMRR-21-760-59664 | ZEUS - Effects of ziltivekimab versus placebo on cardiovascular outcomes in participants with established atherosclerotic cardiovascular disease, chronic kidney disease and systemic inflammation |
| NMRR-19-1258-47901 | Semaglutide cardiovascular outcomes trial in patients with type 2 diabetes |
| NMRR-22-02352-2JT | An Open-label, Multicenter, Phase 2 Study of Sacituzumab Govitecan Combinations in First-line Treatment of Patients with Advanced or Metastatic Non-Small-Cell Lung Cancer (NSCLC) Without Actionable Genomic Alterations |
| NMRR-21-1884-61356 | A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Multicenter Study to Assess the Efficacy and Safety of KBP-5074, a Mineralocorticoid Receptor Antagonist, in Subjects with Uncontrolled Hypertension Who Have Moderate or Severe (Stage 3b/4) Chronic Kidney Disease |
| NMRR-20-2801-57010 | A Phase 2b/3 Double-Blind, Randomized, Placebo-Controlled Study to Evaluate the Efficacy and Safety of MK-1654 in Healthy Pre-Term and Full-Term Infants |
| NMRR-21-1566-60179 | A Phase 3, Multicenter, Randomized, Partially Blinded, Palivizumab-Controlled Study to Evaluate the Safety, Efficacy and Pharmacokinetics of MK-1654 in Infants and Children at Increased Risk for Severe RSV Disease |
| NMRR-19-1288-48307 | A Randomized, Double-blind, Parallel Group, Equivalence, Multicenter Phase III Trial to Compare the Efficacy, Safety,Pharmacokinetics and Immunogenicity of HD204 to Avastin® in patients with Metastatic or Recurrent Non-squamous Non-small Cell Lung Cancer |
| NMRR-19-3681-52367 | A phase III, randomized, double-blind study to assess the efficacy and safety of lazertinib versus gefitinib as the first-line treatment in patients with epidermal growth factor receptor sensitizing mutation positive, locally advanced or metastatic non-small cell lung cancer |
| NMRR-18-3266-45336 | Phase 3, Randomized, Open-label, Controlled, Multiple-Dose, Efficacy, Safety, Pharmacokinetic,and Pharmacodynamic Study of Etelcalcetide in Pediatric Subjects 28 days to < 18 Years of age With Secondary Hyperparathyroidism and Chronic Kidney Disease Receiving Maintenance Hemodialysis |
| NMRR-22-02353-FTQ | A multicenter, international, randomized, placebo controlled, double-blind, parallel group and event driven Phase 3 study of the oral FXIa inhibitor asundexian (BAY 2433334) for the prevention of ischemic stroke in male and female participants aged 18 years and older after an acute non-cardioembolic ischemic stroke or high-risk TIA |
| NMRR-23-00644-HN1 | A Phase 3, Randomized, Double-Blind, Placebo-controlled, Event-driven Study to Demonstrate the Efficacy and Safety of Milvexian, an Oral Factor XIa Inhibitor, After a Recent Acute Coronary Syndrome |
| NMRR-22-01341-1B9 | An Open-label Extension Study to Evaluate Long-term Efficacy and Safety of Odevixibat (A4250) in Children with Biliary Atresia (BOLD-EXT) |
| NMRR-23-01854-V44 | A Phase III, Randomised, Open-Label Study of Savolitinib in Combination With Osimertinib Versus Platinum-Based Doublet Chemotherapy in Participants With EGFR Mutated, MET-Overexpressed and/or Amplified, Locally Advanced or Metastatic Non-Small Cell Lung Cancer Who Have Progressed on Treatment With Osimertinib (SAFFRON). |
| NMRR-23-00646-STW | A phase II, open-label, single-arm, multi-centre study to evaluate the safety and efficacy of osimertinib with amivantamab as first-line treatment in participants with epidermal growth factor receptor mutation-positive, locally advanced or metastatic non-small cell lung cancer (OSTARA). |
| NMRR-24-01400-JD3 | A Phase IIb, Multicenter, Randomised, Double-Blind, Dose-finding Study to Evaluate the Efficacy, Safety and Tolerability of Balcinrenone in Combination With Dapagliflozin Compared With Dapagliflozin in Patients With Chronic Kidney Disease and Albuminuria |
| NMRR-23-02718-WNX | A Randomised, Double-Blind, Placebo-Controlled, Parallel Group Study to Assess the Efficacy and Safety of Baxdrostat in Participants With Uncontrolled Hypertension on Two or More Medications Including Participants With Resistant Hypertension |
| NMRR-24-00421-VYY | A Randomised, Double-Blind, Placebo-Controlled, Parallel Group Study to Assess the Effect of Baxdrostat on Ambulatory Blood Pressure in Participants with Resistant Hypertension |
| NMRR-19-33-45674 | A Phase 3, Multicenter, Open-Label Extension Study to Evaluate the Long-Term Efficacy and Safety of Mirikizumab in Patients with Moderately to Severely Active Ulcerative Colitis |
| NMRR-18-809-41239 | A Phase 3, Multicenter, Randomized, Double-Blind, Parallel-Arm, Placebo-Controlled Maintenance Study of Mirikizumab in Patients with Moderately to Severely Active Ulcerative Colitis |
| NMRR-23-02546-OLS | A Phase 2A Randomised, Double-Blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of AZD4604 Twice Daily for Twelve Weeks in Adult Patients With Moderate-to-Severe Asthma Uncontrolled on Medium-High Dose ICS-LABA |
| NMRR-23-02850-UTZ | A Phase III, Multicentre, Randomised, Double-blind, Chronic-dosing, Parallel-group, Placebo-controlled Study to Evaluate the Efficacy and Safety of Tozorakimab in Participants With Symptomatic Chronic Obstructive Pulmonary Disease (COPD) With a History of COPD Exacerbations (MIRANDA) |
| NMRR-24-00104-JLP | ARTEMIS - Effects of Ziltivekimab Versus Placebo on Cardiovascular Outcomes in Patients With Acute Myocardial Infarction |
| NMRR-23-00677-TWB | A Global, Phase 3, Randomized, Multicenter, Open-Label Study to Investigate the Efficacy and Safety of Furmonertinib Compared to Platinum-Based Chemotherapy as First-Line Treatment for Patients With Locally Advanced or Metastatic Non-Small Cell Lung Cancer (NSCLC) With Epidermal Growth Factor Receptor (EGFR) Exon 20 Insertion Mutations (FURVENT) |
| NMRR-21-1613-60813 | A Randomized Open-Label, Phase 3 Study to Evaluate Imetelstat (GRN163L) Versus Best Available Therapy (BAT) in Patients with Intermediate-2 or High-risk Myelofibrosis (MF) Refractory to Janus Kinase (JAK) Inhibitor |
| NMRR-23-02720-YHF | A Randomized, Open-label, Phase 3 Study of MK-2870 vs Chemotherapy (Docetaxel or Pemetrexed) in Previously Treated Advanced or Metastatic Nonsquamous Non-small Cell Lung Cancer (NSCLC) With EGFR Mutations or Other Genomic Alterations |
| NMRR-23-00029-QKE | An Open-label, Multicenter, Phase 3 Randomized, Active-Comparator-Controlled Clinical Study of Pembrolizumab (MK-3475) in Combination With Sacituzumab Govitecan Versus MK-3475 Monotherapy as First-line Treatment in Participants With PD L1 TPS Greater Than or Equal to 50% Metastatic Non-small Cell Lung Cancer (KEYNOTE D46/EVOKE-03) |
| NMRR-23-03442-6BH | Effects of Ziltivekimab Versus Placebo on Heart Failure Symptoms and Physical Function in Patients With Heart Failure With Mildly Reduced or Preserved Ejection Fraction and Systemic Inflammation |
| NMRR-23-03240-K1D | An Open-label, Dose-escalation, Dose-finding, and Proof-of-concept Trial of SP-420 in Subjects With Transfusion-dependent ß-thalassemia |
| NMRR-23-02859-7SS | A Phase III Randomised, Double-blind, Multicentre Study to Compare the Efficacy, Safety, Pharmacokinetics, and Immunogenicity between SB27 (proposed pembrolizumab biosimilar) and Keytruda in Subjects with Metastatic Nonsquamous Non-small Cell Lung Cancer. |
Meet Our Collaborators

Upcoming Activities

CRC Hospital Raja Perempuan Zainab II, Kota Bharu, Kelantan
Email: crckelantan@gmail.com