EVOS
Early Versus Onward Switching
Evaluating the non-inferiority and safety of an early switch from intravenous (IV) to oral antibiotics compared to the standard 14-day IV antibiotic regimen for patients with uncomplicated Staphylococcus Aureus Bacteraemia (SAB).
Study No: NCT06336824
Information provided by: Steven Lim Chee Loon, Ministry of Health, Malaysia
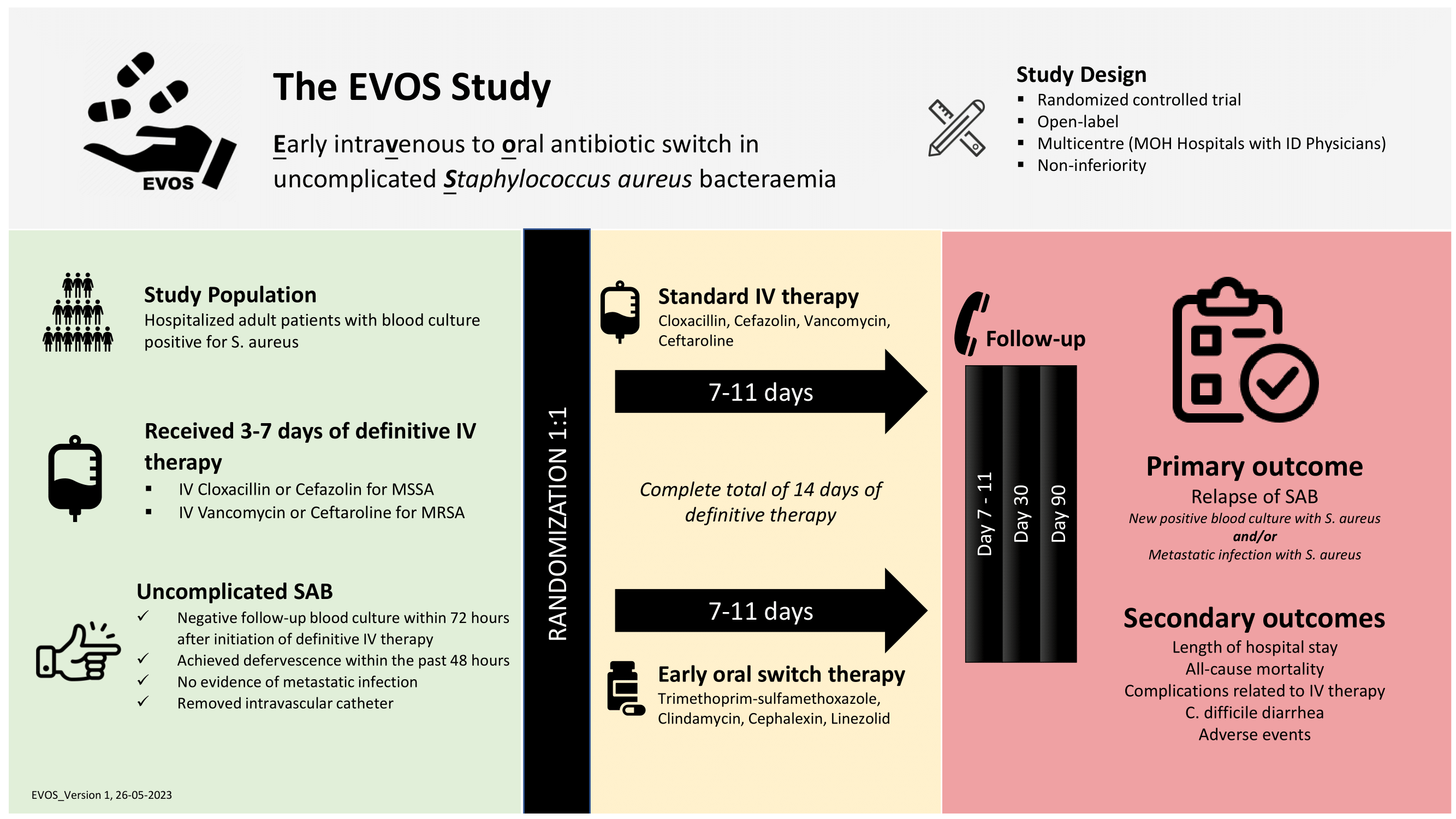
Brief Summary: The Early Intravenous to Oral Antibiotic Switch in Uncomplicated Staphylococcus aureus Bacteraemia (EVOS) study is a multicentre, randomized, open-label, parallel group, phase 3, non-inferiority trial of early intravenous to oral antibiotic switch in comparison with standard intravenous antibiotic regime among patients with uncomplicated Staphylococcus aureus bacteraemia (SAB). The study is based on the hypothesis that an early switch from IV to oral antimicrobial therapy is non-inferior and safe compared to conventional minimum 14-day course of IV therapy in patients with low-risk uncomplicated SAB.
Detailed Description: The study is conducted at 12 government tertiary hospitals with infectious diseases physicians in Malaysia. The study population comprises of 290 patients with uncomplicated SAB who have received 3 to 7 days of definitive IV antimicrobial therapy. Eligible participants are randomized 1:1 into 2 groups, early oral antibiotic switch versus standard IV antibiotic therapy, following the inclusion and exclusion criteria.
The study consists of 3 stages for each patient with a duration of approximately 12 weeks: screening and enrolment, open-label treatment with 7 to 11 days of study antibiotics, and follow-up until day 90 post-randomization. Phone call or inpatient follow up will be conducted at Day 7-11, Day 30, and Day 90 post- randomization to review patient's condition
Condition/Disease: Staphylococcus Aureus Bacteremia
Intervention / Treatment: Drug: Tab. Trimethoprim-sulfamethoxazole, Tab. Clindamycin, Tab. Cephalexin, or Tab. LinezolidDrug: IV Cloxacillin, IV Cefazolin, IV Vancomycin, or IV Ceftaroline
Study Start: 05-2024 (Estimated)
Primary Completion: 05-2025 (Estimated)
Study Completion: 06-2026 (Estimated)
Enrollment: 290 (Estimated)
Study Type: Interventional (Clinical Trial)
Study Phase: Phase 3
Investigator site:
Study Contact:
Dr. Steven Lim
+60133620081
stevenlimcl@gmail.com
Dr. Josephine P Durai
+6052085146
joeyukijo.28@gmail.com
Sponsor: Ministry of Health Malaysia (MOH) grant
Collaborator: Clinical Research Centre (CRC)