WhoWe Are
Our Location
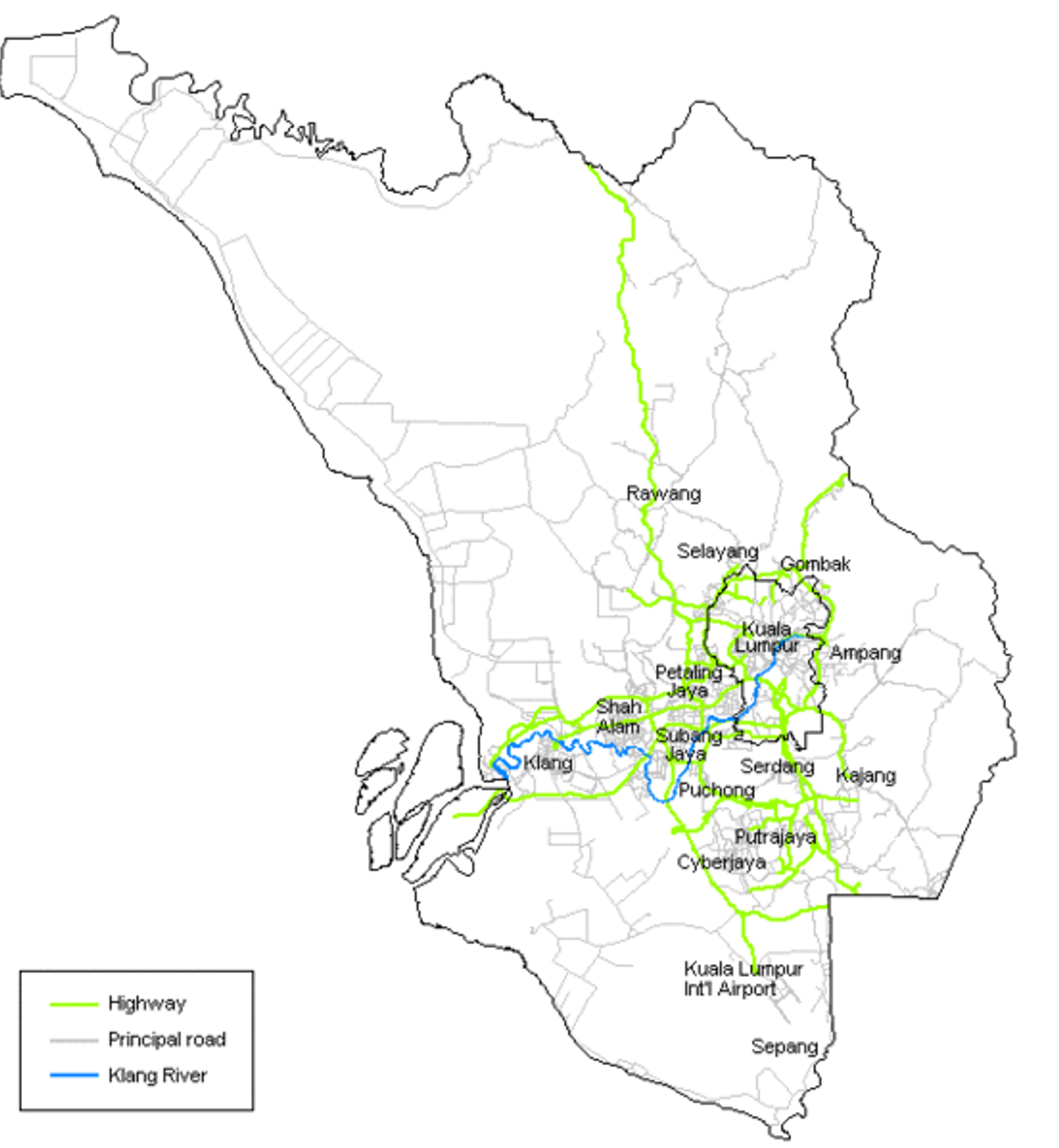

Hospital Ampang
- Tertiary hospital with 562 beds capacity
- National referral centre for haematology
- Medical, Emergency and ICU support
- Within Klang Valley
- 25% of total population (~8.2mil)
- Highly accessible
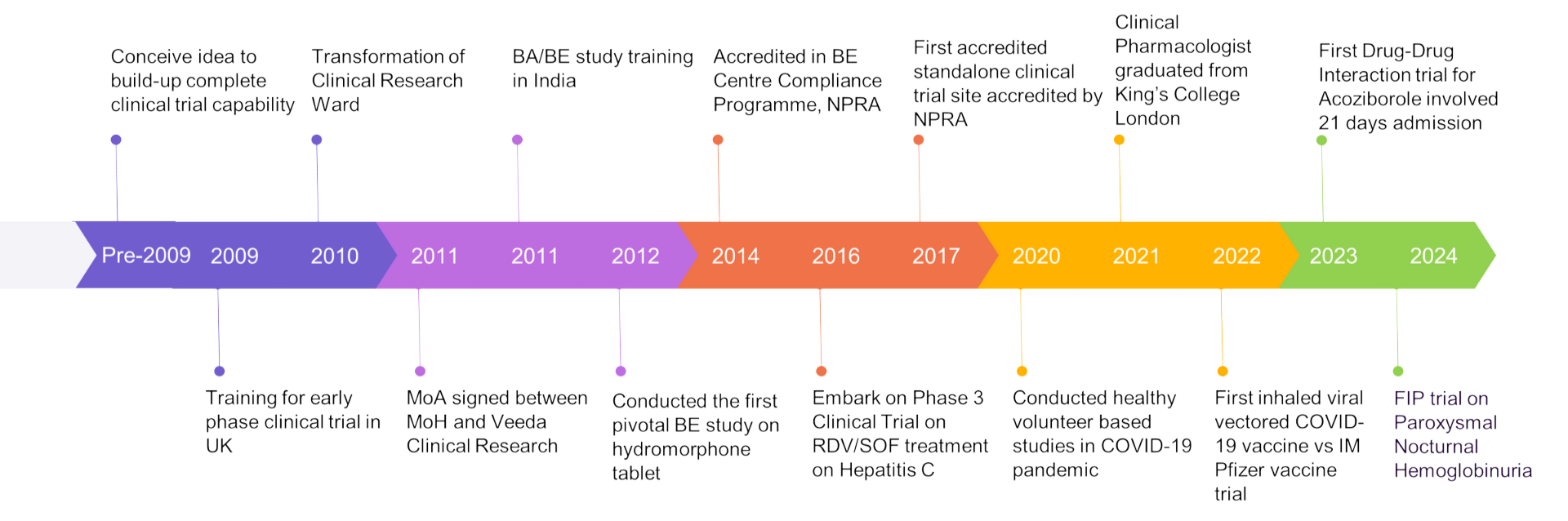
Centre for Clinical Trial (CCT) was established in 2010 to conduct and support early and late phase clinical research in Malaysia. Besides early-phase clinical research, we also conduct bioavailability (BA) and bioequivalence (BE) studies for pharmaceutical industries in accordance with local and international standards. Our research facility, Clinical Research Ward (CRW) is located at Ampang Hospital, the largest referral centre for haemato-malignancies in Malaysia. Clinical studies conducted at CRW are closely managed and monitored to ensure adherence to international and local Good Clinical Practice standards. CRW was accredited by the National Pharmaceutical Regulatory Agency (NPRA) in 2014 as a BA/BE compliance site, in accordance with the Ministry of Health Malaysia Compliance Programme for Bioequivalence Centre.
Vision:
- Being a Catalyst That Ensures Clinical Research Centers Conduct Ethical and Quality Clinical Research.
Mission:
- To ensure the conduct of ethical and high-quality clinical research that contributes to the advancement of medical knowledge and benefits the patients.
Our Experiences:
- Accredited Bioequivalence Centres in Bioequivalence Centre Compliance Programme, National Pharmaceutical Regulatory Agency (NPRA) 2014
- First accredited standalone clinical trial site accredited by NPRA, 2017
- First accredited Phase 1 Unit in Peninsular Malaysia by NPRA, 2022
- Inspection for full accreditation of Phase 1 Unit planned on Apr 2024
- First-in-Human trial on Paroxysmal Nocturnal Hemoglobinuria (PNH)
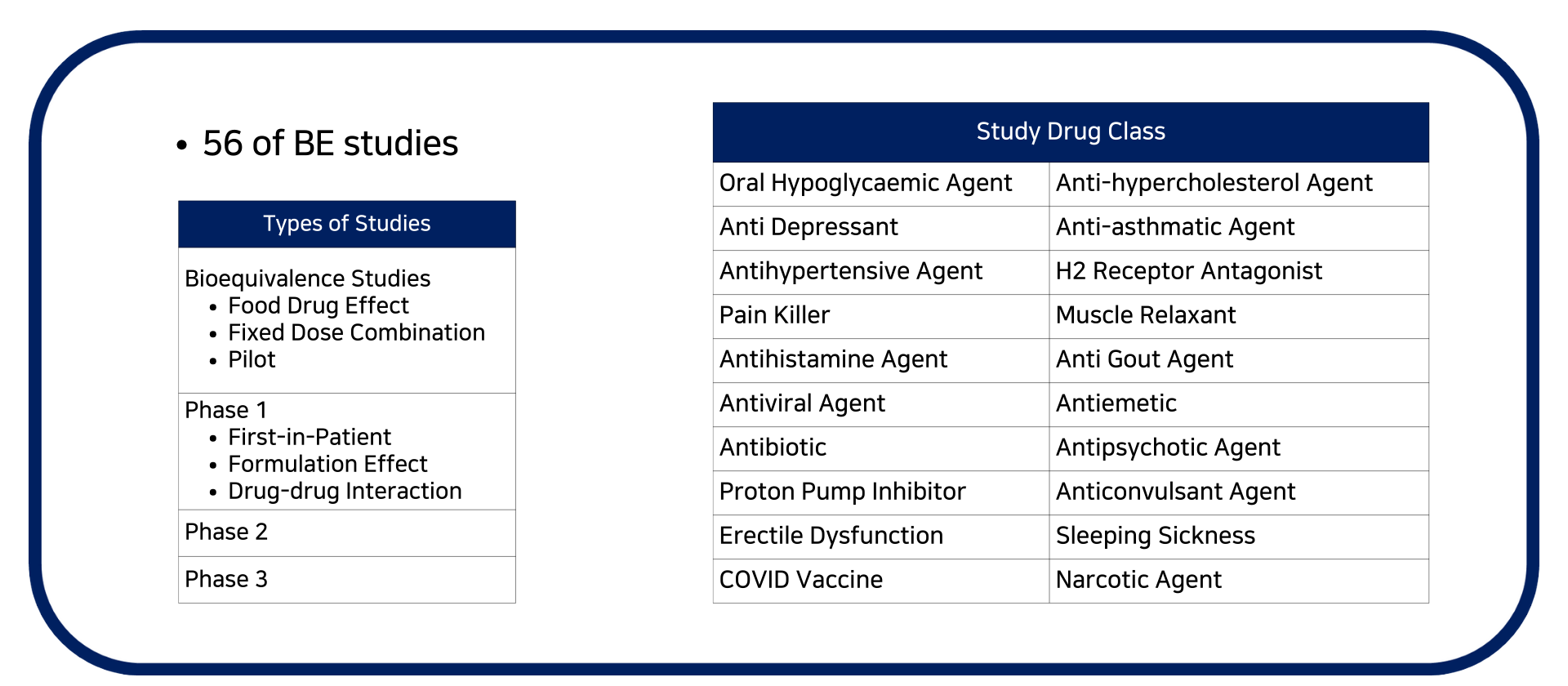
- Bioavailability / Bioequivalence studies For US FDA, EMA, TGA, HSA, Taiwan FDA, NPRA submission
- Food drug effect studies
- Drug-drug interaction study
- Phase 2 and 3 clinical trials
- Inspection by NPRA
- Audited by local and international companies
- Data and safety monitoring board (DSMB) for other clinical trials
Over 10 years of dedication to Clinical Trial
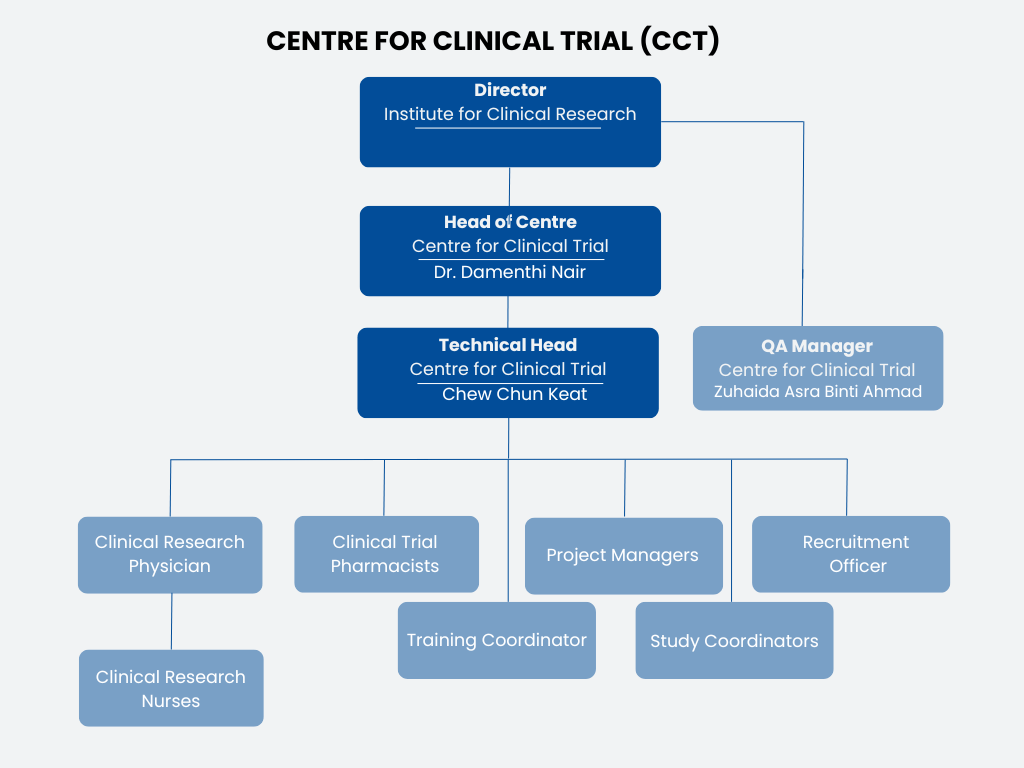
OrganizationChart
InvestigatorsHighlight

Head, Centre for Clinical Trial (CCT)
Email: damenthi@moh.gov.my
MD
Areas of interest: Healthy Volunteer Research, First in Human Trials
Dr. Damenthi Nair is the Head of the Center for Clinical Trials (CCT) at Hospital Ampang. She has extensive experience in leading bioequivalence (BE) studies and has served as a principal investigator for various trials, including the Phase 1 Ravidasvir DNDi study. She has fostered partnerships with local and international companies for the execution of clinical trials, including involvement in Covid Vaccine Trials. Dr. Damenthi Nair is currently engaged in a collaborative effort to spearhead the development and implementation of Phase 1, 2, and 3 trials at Hospital Ampang.
Research Highlights
- FIH- Phase 1 study: An Open-Label, Multicenter, Intra-Subject Dose Escalation Study to Evaluate the Safety, Tolerability, Pharmacokinetics, Pharmacodynamics, and Therapeutic Potential of BCX10013 in Subjects with Paroxysmal Nocturnal Hemoglobinuria
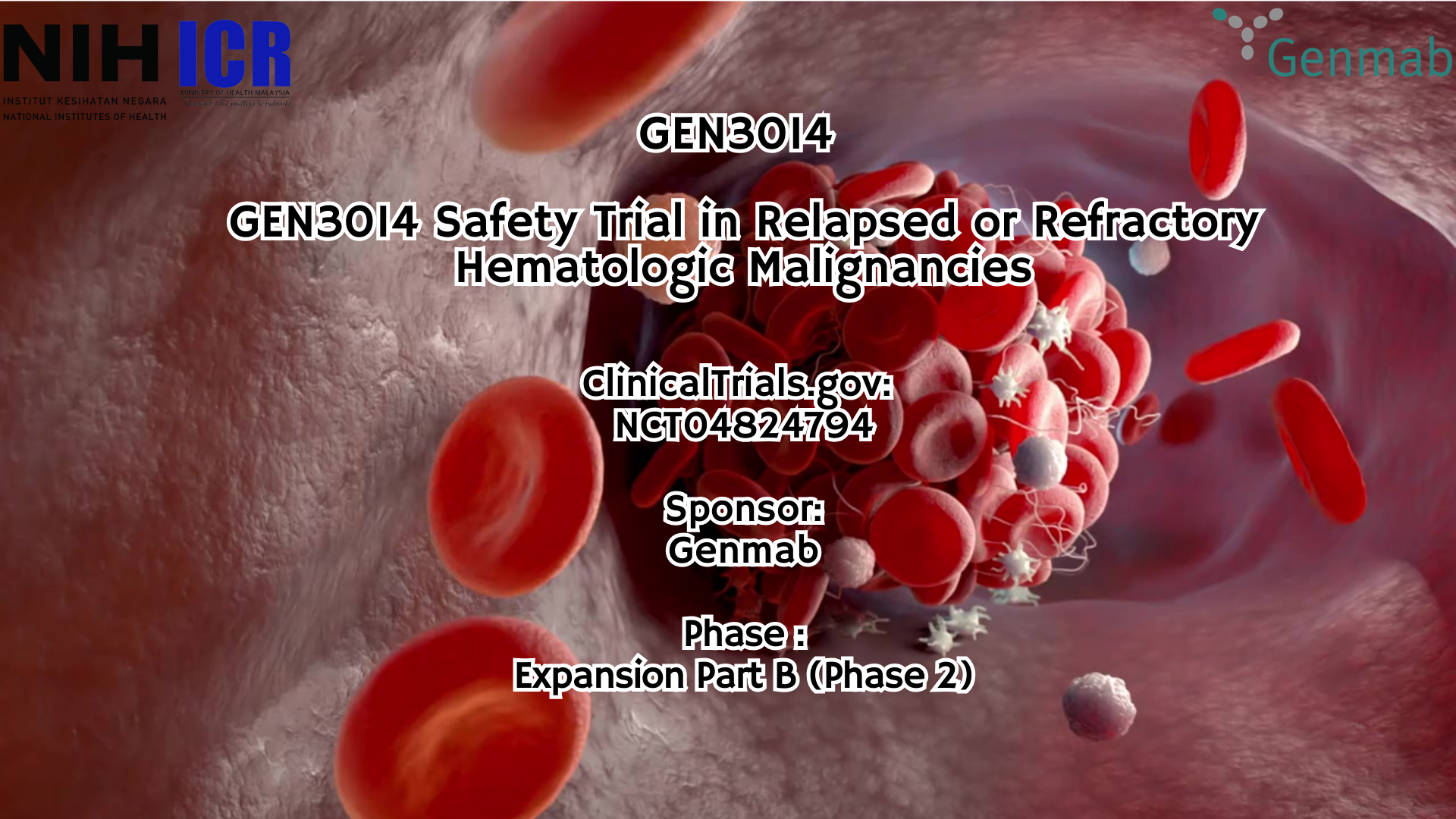
- An Open-Label, Multicenter, Phase 1/2 Trial of GEN3014 (HexaBody®-CD38) in Relapsed or Refractory Multiple Myeloma and Other Hematologic Malignancies
Recent Investigator Sponsored Research
Study No: DNDi-OXA-07-HAT
Study Phase: Phase I (Drug-drug interactions)
Study Title: A single centre open-label, non-randomised, three-treatment, two-period, pharmacokinetic drug interaction study of single oral dose of acoziborole with sequential co-administration of midazolam and dextromethorphan in healthy male participants
Study No: DNDi-SOF/RDV-01-HCV
Study Phase: Phase II/III
Study Title: Open label phase II/III, multicenter, trial to assess the efficacy, safety, tolerance, and pharmacokinetics of sofosbuvir plus ravidasvir in HCV (+/- HIV) chronically infected adults with no or compensated cirrhosis in Thailand and Malaysia
Study No: 20200404 (IMBCAMS)
Study Phase: Phase III
Study Title: Randomized, Double -Blinded, Placebo Controlled Phase III Clinical Trial for the Evaluation of Efficacy and Safety of SARS-CoV-2 Vaccine, Inactivated (Vero Cell) in Healthy Population Aged 18 Years and Above in Malaysia
Study No: Cansino Bio Vaccine Trial CT 2022-01
Study Phase: Phase III (Non-Inferiority Trial)
Study Title: Immunogenicity, Efficacy and Safety of Inhaled (IH) viral vectored vaccine (Convidecia, CanSino) as second booster dose against emerging Variants of Concern (VOC) of SARS-CoV-2 to prevent breakthrough infections. A randomized observer-blind controlled trial.

Technical Head, Centre for Clinical Trial (CCT)
Email: chewchunkeat@moh.gov.my
Master in Clinical Research Methods (MCRM), Bachelor of Pharmacy (Honour) [BPharm (Hon)], Diploma in Computer Science
Areas of interest: Early phase clinical trials, bioavailability/bioequivalence studies, study methodology, pharmacovigilance, bioethics.
Chew Chun Keat (CK), is the Technical Head of Centre for Clinical Trial. He is a pharmacist with a Master in Clinical Research Methods from Monash University and a Diploma in Computer Studies. He trained in phase 1 clinical trials in the UK and India and helped develop a Phase 1 Clinical Trial Unit at Hospital Ampang. The unit has conducted many pharmacokinetic/pharmacodynamic studies determining bioavailability of study compound, drug-drug interaction, food drug effect, drug formulation effect, bioequivalence studies, first-in-patient and vaccine trials. He has established the National Healthy Research Volunteers Register (NHRVR). CK has contributed to several Malaysian clinical trial guidelines. He is a board member of the Medical Research and Ethics Committee since 2015
Research Highlights
We are part of the WHO Solidarity Trial where it is a major international clinical trial involving 52 countries to evaluate COVID-19 treatments. Initially testing remdesivir, hydroxychloroquine, lopinavir/ritonavir, and lopinavir/ritonavir with interferon beta-1a, the trial uses an adaptive design for flexibility. Interim results in October 2020 showed these treatments did not significantly reduce mortality or ventilation needs in hospitalized patients. The trial's large scale and rapid data generation have been crucial in informing global health policies and treatment guidelines. It continues to evaluate new therapies, demonstrating the power of global collaboration in addressing the pandemic.
Recent Publications

Clinical Pharmacologist
Email: sharonng@moh.gov.my
MBBS (MMMC), MSc Clinical Pharmacology (KCL)
Areas of interest: Healthy volunteer research, first-in-human trials
Awards and achievements:
- CRM Sponsored Research Award for “Top Recruiter” 2023
Dr. Sharon Ng is a highly experienced principal investigator who specializes in conducting research on healthy volunteers. She completed her qualification as a clinical pharmacologist at Kings College London with distinction and has experience in First-In-Human trials. In the year 2023, she completed two notable clinical trials, namely the Drugs for Neglected Diseases initiative (DNDi) drug-drug interactions study that housed subjects for 21 days and a multicentre clinical trial evaluating the safety and efficacy of Convidecia AirTM, the first inhalation COVID-19 vaccine in the world. Her outstanding work in the latter trial won her the 2023 Clinical Research Malaysia Sponsored Research Award under the Top Recruiter category. She attributes her success to her team in Hospital Ampang who played a fundamental in conducting the clinical trials.
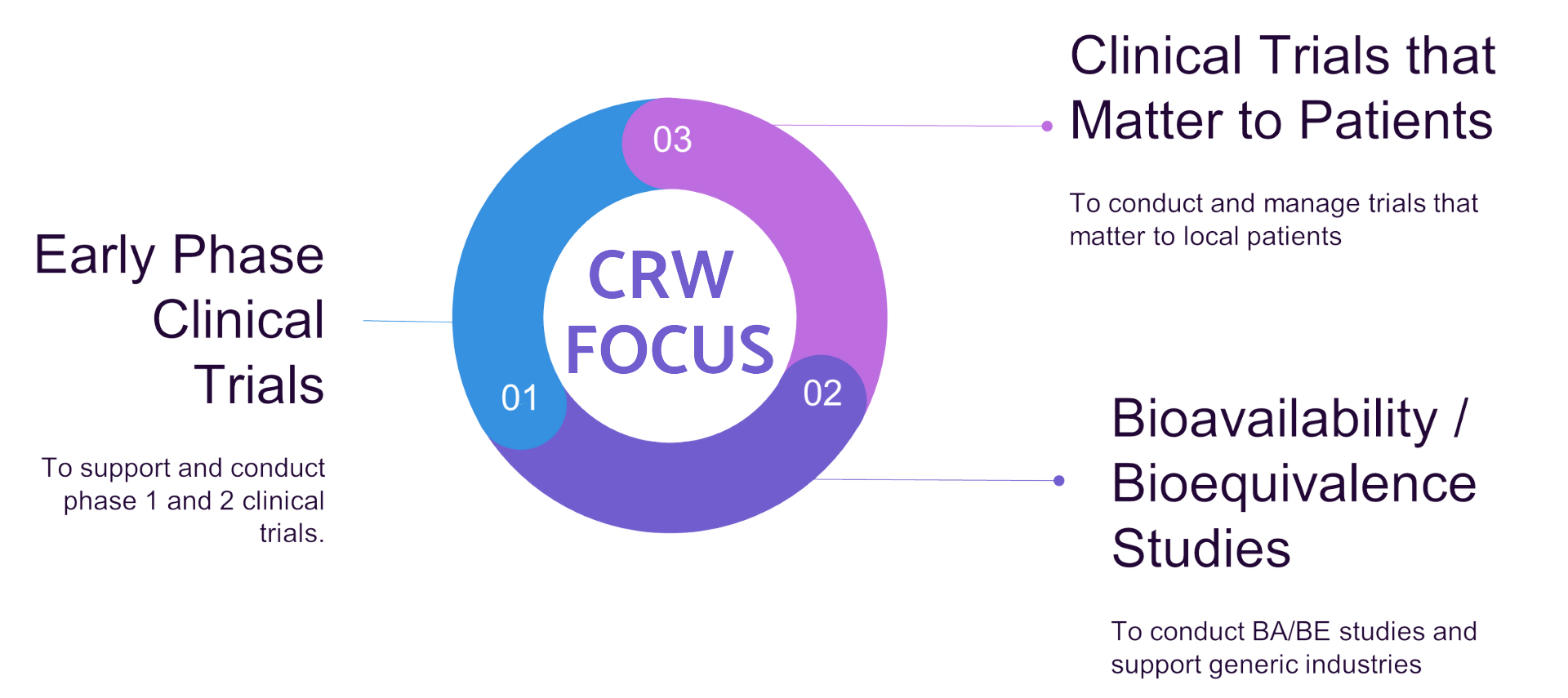
Services at Centre for Clinical Trial
Clinical Research Ward
- Protocol development.
- Informed consent documents development.
- Case report form design.
- Ethics submission.
- Regulatory submission.
- Volunteer recruitment (Health volunteers and patients).
- Clinical conduct.
- Project management.
Phase 1 Ward
10 beds equipped with central monitoring system
Remote monitoring assess (CCTV monitored)
BE Ward Area
4 open bay with total of 36 beds
Screening Room
2 rooms for informed consent taking and screening procedures
Recreational Room
A space for volunteers to relax
Research Highlights
Study Phase: Phase I (Drug-drug interactions)
Study Title: A single centre open-label, non-randomised, three-treatment, two-period, pharmacokinetic drug interaction study of single oral dose of acoziborole with sequential co-administration of midazolam and dextromethorphan in healthy male participants
Study Indications: Human African Trypanosomiasis (HAT)
Investigational Product: Acoziborole
Arms & Intervention / Treatment: Acoziborole
Sponsor/Co-Sponsors: DNDi, Chemin Camille-Vidart, 15, 1202 GENEVA Switzerland
Study Phase: Phase II/III
Study Title: Open label phase II/III, multicenter, trial to assess the efficacy, safety, tolerance, and pharmacokinetics of sofosbuvir plus ravidasvir in HCV (+/- HIV) chronically infected adults with no or compensated cirrhosis in Thailand and Malaysia
Study Indications: Chronic Hepatitis C infection [+/- HIV co-infection]
Investigational Product: Sofosbuvir 400 mg tablet manufactured by European Egyptian Pharmaceutical Industries (EEPI), Egypt
Arms & Intervention / Treatment: Sofosbuvir 400 mg tablet vs Ravidasvir 200 mg
Sponsor/Co-Sponsors: DNDi, Chemin Louis Dunant, 15, 1202 GENEVA Switzerland, Ministry of Health Malaysia and Ministry of Public Health Thailand
Study Phase: Phase III
Study Title: Randomized, Double -Blinded, Placebo Controlled Phase III Clinical Trial for the Evaluation of Efficacy and Safety of SARS-CoV-2 Vaccine, Inactivated (Vero Cell) in Healthy Population Aged 18 Years and Above in Malaysia
Study Indications: To prevent diseases caused by SARS CoV-2 infection
Investigational Product: SARS-CoV-2 Vaccine, Inactivated (Vero Cell) that developed by Institute of Medical Biology Chinese Academy of Medical Sciences is used as the investigational vaccine.
Arms & Intervention / Treatment: SARS-CoV-2 Vaccine vs Placebo
Sponsor/Co-Sponsors: Institute of Medical Biology, Chinese Academy of Medical Sciences
Study Phase: Phase III (Non-Inferiority Trial)
Study Title: Immunogenicity, Efficacy and Safety of Inhaled (IH) viral vectored vaccine (Convidecia, CanSino) as second booster dose against emerging Variants of Concern (VOC) of SARS-CoV-2 to prevent breakthrough infections. A randomized observer-blind controlled trial.
Study Indications: To induce protective immunity in a vaccinee against a targeted infectious pathogen such as the SARS-Cov-2 virus
Investigational Product: IH Convidecia, a recombinant Novel Coronavirus Vaccine, Adenovirus Type 5 Vector, manufactured by CanSino Biologics Inc. China/ Solution Biologics Bhd Malaysia.
Arms & Intervention / Treatment: IH Convidecia vaccine vs mRNA vaccine BNT162b2 (Pfizer)
Sponsor/Co-Sponsors: CanSino Biologics Inc, 185 South Ave., TEDA West District, Tianjin, China
Meet Our Collaborators


























Centre for Clinical Trial (CCT)
Clinical Research Ward,
Level 7, Hospital Ampang
Jalan Mewah Utara,
Pandan Mewah
68000 Ampang, Selangor
Main Phone: +603-42896558 | Fax: 03-42801409
Email: ctuampang@moh.gov.my